BACKGROUND: Anti-B-cell maturation antigen (BCMA) chimeric antigen receptor T-cell (CART) therapy shows remarkable efficacy in patients with relapsed and/or refractory (R/R) multiple myeloma (MM), with recent phase 3 studies showing clear advantage of idecabtagene vicleucel (ide-cel) and ciltacabtagene autoleucel (cilta-cel)over standard-of-care. However, access to these commercial CART products is a major barrier, and it is estimated that only around a quarter of eligible R/R MM patients will receive CART nowadays.
HBI0101 is a novel second generation optimized anti-BCMA CART with 4-1BB co-stimulatory domain, that was developed in an academic setting, at Hadassah Medical Center and Bar-Ilan University. The phase Ia study evaluating HBI0101 (NCT04720313) demonstrated a manageable safety profile and an initial high efficacy (Asherie, Haematologica 2022). Here, we present the updated results of 50 patients receiving 800x10^6 CART cells in the NCT04720313 phases 1a-b / 2.
METHODS: The phase 1a study with HBI0101 which first evaluated 20 R/R MM patients with ≥3 prior lines of therapy, including a PI, IMiD and anti-CD38 antibody, revealed a dose of 800x10^6 CART cells to be safe and effective. The phase Ib and phase 2 of the study evaluated this dose infusion in 43 additional patients. Inclusion criteria were relatively permissive with minimum creatinine clearance of 20ml/min, platelet count of 30x10 9/ml, left ventricular ejection fraction of 40%, and ECOG performance status of ≤2. Lymphodepletion included fludarabine 25mg/m 2 and cyclophosphamide 250mg/m 2 on days -5 to -3 before infusion (bendamustine 90mg/m 2 and on days -4 and -3 for patients with creatinine clearance <30ml/min). Planned manufacturing time was 10 days.
RESULTS: Of 51 patients who underwent lymphocyte apheresis, 50 (98%) were treated with 800x10^6 CART cells; 7 patients in the phase Ia cohort and 43 in the phases 1b / 2 cohorts. HBI0101 production success rate was 100%, with manufacturing time of 10 days for all patients. The median age was 65y (range 40-84). The median number of prior lines was 4 (range 3-13) and most patients (47/50, 94%) were triple refractory to PI, IMiD and anti-CD38 antibody. Twenty patients (40%) were penta-refractory and 10 (20%) were refractory to the anti-BCMA antibody drug conjugate belantamab mafodotin. Forty-six patients (92%) were refractory to the last line of therapy. Twelve patients (24%) had high risk cytogenetics (t(4:14), t(14:16) or deletion 17p); 31 (62%) when 1q gain included. Sixteen patients (32%) had extramedullary involvement at study entry.
Grade 3-4 hematological toxicities were common (anemia- 62%, thrombocytopenia- 54%, neutropenia- 98%, lymphopenia- 100%). Cytokine release syndrome (CRS) occurred in 48/50 (96%)- grade 1/2- 17 patients (82%); grade 3- 7 patients (14%). Tocilizumab was used in 40/48 patients with CRS (median of 1 dose, range 1-4) and corticosteroids in 8/48. Two cases of immune effector cell associated neurotoxicity syndrome (ICANS) were observed (grade 1 and 2). Two patients developed CART-associated hemophagocytic lymphohistiocytosis syndrome (HLH). No irreversible organ toxicities or treatment related deaths occurred.
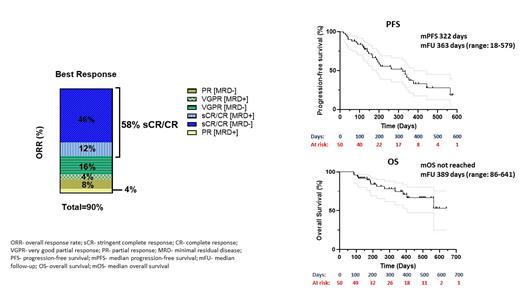
The overall response rate (ORR) was 45/50 (90%), including 29 patients (58%) with complete response (CR)/stringent CR, 10 (20%) with very good partial response and 6 (12%) with partial response. Thirty-five patients (70%) achieved minimal residual disease (MRD) negativity at day +30. At a median follow-up of 11.9 months (range: 0.6-19), the median progression-free survival was 10.6 months, and the median overall survival was not reached (Figure 1).
Although the presence of extra-medullary disease and prior anti-BCMA therapy correlated with worse outcome, high response rates were still observed (ORR of 98%/75% for patients without/with extra-medullary disease, respectively, and 95%/75% for patients without/with prior anti-BCMA therapy, respectively). Updated results will be communicated at the presentation time.
CONCLUSION: Our findings demonstrate the manageable safety and high efficacy profiles of HBI0101 also in high-risk patients. Fast production time enabled the timely treatment of 98% of apheresed patients. These favorable data are encouraging and support decentralization of CART production at an academic setting, ensuring a sufficient CART supply in the light of the increasing demand.
Disclosures
Cohen:Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; GSK: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Avivi Mazza:AbbVie: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal